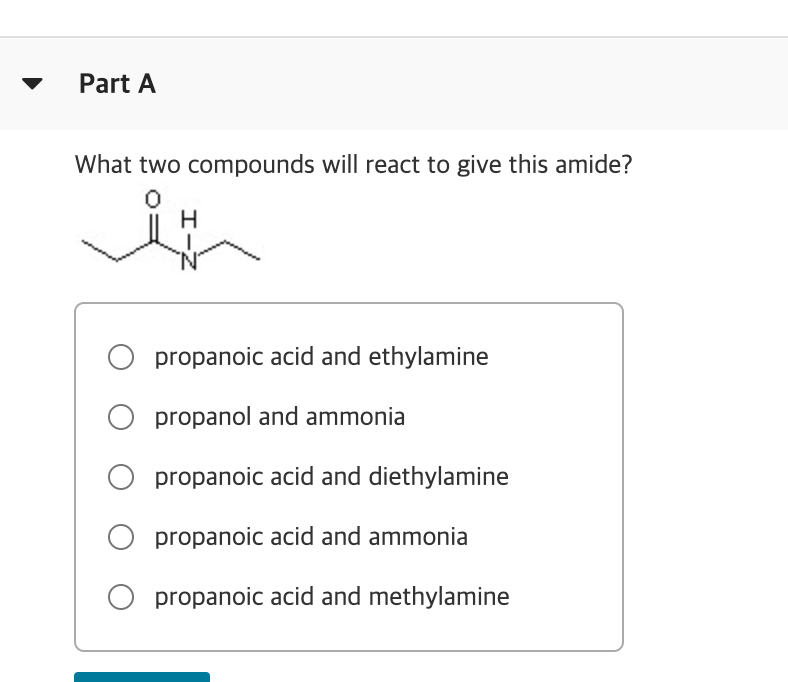
What Two Compounds Will React to Give This Amide
A ketones reaction with ammonia followed by sodium cyanoborohydrides catalytic reduction or degradation produces a 1 amine. Iii Primary amines on heating with chloroform and ethanolic KOH foul-smelling substances known as isocyanides or carbylamines are formed.

Amines And Amides Chemistry For Majors
Among the amides of commercial importance are acetamide also called ethanamide CH 3 CONH 2 and dimethylformamide HCONCH 3 2 which are used as solvents the sulfa drugs and the nylons.
. Primary amine reacts with CHCl 3 KOH to form isocyanides carbylamines. Just as the reaction of a diol and a diacid forms a polyester Section 158 Preparation of Esters the reaction of a diacid and a diamine yields a polyamide A condensation polymer in which the monomer units are joined by an amide linkageThe two difunctional monomers often employed are adipic acid and 16-hexanediamine. You get a very unpleasant smell with the.
Aromatic amines react with nitrous acid HNO 2 at low temperature 273-278 K to form diazonium salts. A The Reaction of 1 o Amines b The Reaction of 2 o Amines c The Reaction of 3 o Amines. The addition of ammonia NH 3 to a carboxylic acid forms an amide but the reaction is very slow in the laboratory at room temperature.
Acid chlorides react with ammonia to give amides also by an addition-elimination path and these are reduced to amines by LiAlH 4. Show transcribed image text Expert Answer. Ii Nitrocompounds on reduction gives amines.
Experts are tested by Chegg as specialists in their subject area. Amines are organic derivatives of ammonia in which one two or all three of the hydrogens of ammonia are replaced by organic groups. Describe the preparation procedure for amides.
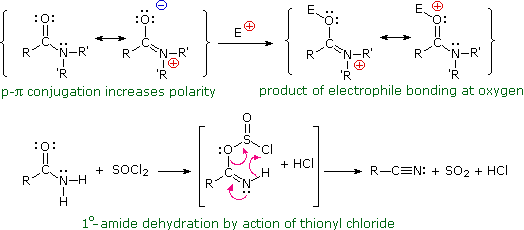
To describe the preparation procedure for amides. If we take an amide and react it with thionyl chloride SOCl 2 the organic product is a nitrile carbon-nitrogen triple bond. Reduction of Nitriles Amides and Nitro Compounds Acid chlorides react with ammonia to give amides by an addition-elimination path and these are reduced to amines by LiAlH 4 Section 21-7.
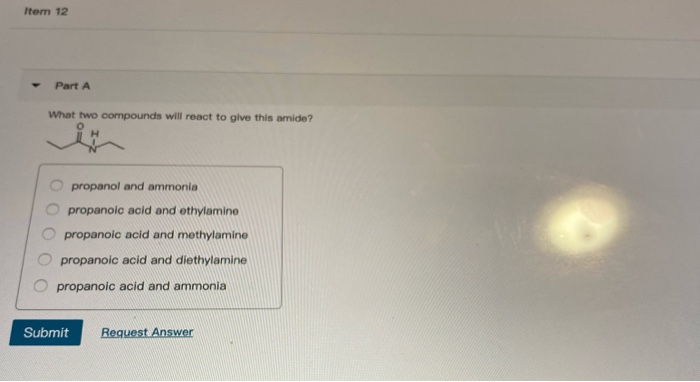
An ethyl line structure with an oxygen double bonded to it and a Hydrogen and Nitrogen bonded to it propanoic acid and ethylamine. The 6th example is a specialized procedure for bonding an amino group to a 3º-alkyl group none of the previous methods accomplishes this. Three classes of amines react differently with nitrous acid as follows.
We have discovered that the camphor-derived amide-stabilized ylide reacts with aldehydes at -50 degrees C in ethanol to give glycidic amides in one step with up to 99 ee and complete diastereoselectivity. Water molecules are split out and a bond is formed between the nitrogen atom and the carbonyl carbon atom. The addition of ammonia NH 3 to a carboxylic acid forms an amide but the reaction is very slow in the laboratory at room temperature.
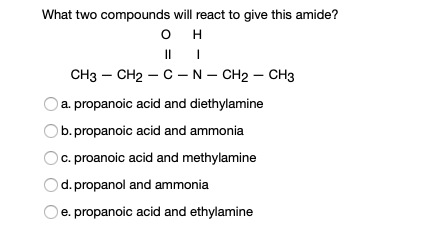
We also get sulfur dioxide gas and HCl gas as by-products and for. What two compounds will react to give this amide. Exercise 24-39 For each of the following pairs of compounds give a chemical test preferably a test-tube reaction that will distinguish the two compounds.
The process is known as diazotisation eg. In living cells amide formation is catalyzed by. The designation of amines as primary secondary and tertiary is different from the usage.
This problem has been solved. In living cells amide formation is catalyzed by enzymes. What two compounds will react to give this amide.
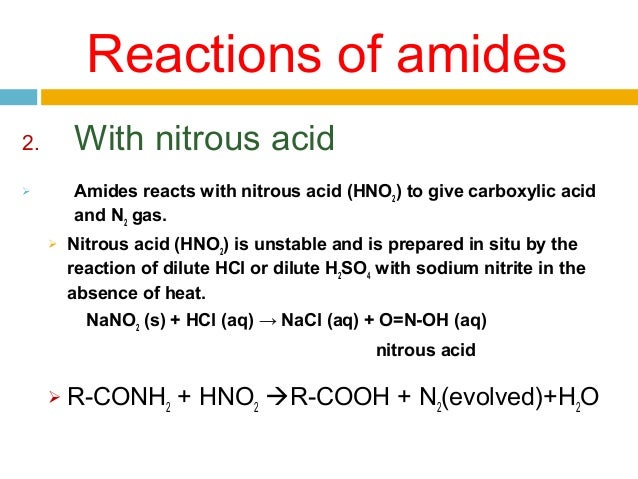
Chemical Reactions of Amines 1 Reaction with Nitrous Acid HNO 2. What two compounds will react to give this amide. Water molecules are split out and a bond is formed between the nitrogen atom and the carbonyl carbon atom.
Who are the experts. The alkyl halides and sulfonates used in this reaction are primary or unbranched secondary. The reaction of an amide-stabilized sulfonium ylide bearing chiral groups on sulfur has been investigated.
See the answer See the answer See the answer done loading. Alkyl halides can be converted to primary amines through a. Catalytic or chemical reactions in the presence of ammonia or primary or secondary amines may eliminate aldehydes or ketones resulting in primary secondary or tertiary amines.
In this reaction an acid amide is heated with Br 2 and aq. Compounds RNH 2 are called primary amines R 2 NH secondary amines and R 3 N are tertiary amines. Write a structural formula for each compound and equations for the reactions involved.
This involves migration of alkyl or aryl group from carboxyl. N O propanoic acid and ammonia O propanoic acid and methylamine O propanol and ammonia O propanoic acid and ethylamine O propanoic acid and diethylamine. NaOH when 1 amine having one carbon atom less is produced.
Amines React as Bases in Water Ammonia NH3 acts as a Brønsted-Lowry base because it accepts H from water to produce an ammonium ion NH4 and a hydroxide ion OH. Reaction of amides with acid chlorides or anhydrides produces imides which are compounds with two carbonyl CO groups attached to the same nitrogen atom. Propanoic acid and methylamine O propanol and ammonia propanoic acid and diethylamine O propanoic acid and ethylamine propanoic acid and ammonia The product of a reaction between CH CHCOOH and CH CH OH will produce View Available Hint s OCHCHCOCHCH H2O OCHCHCOOCH3 H20 OCHCOOCH CH3 H20.
Because the N-alkylated phthalimide formed in this reaction is really a double amide it can be converted into the free amine by amide hydrolysis in either strong acid or base. In water amines act as Brønsted-Lowry bases because the lone electron pair on the nitrogen atom accepts a hydrogen ion from water and produces alkylammonium and hydroxide ions. What two compounds will react to give this amide.
I Aniline reacts with bromine water at room temperature to give a white precipitate of 246 tribromoaniline. 1-methyl-3-nitrobenzene and phenylnitromethane b. We review their content and use your feedback to keep the.
What Are The Reactions For Amines And Amides Example
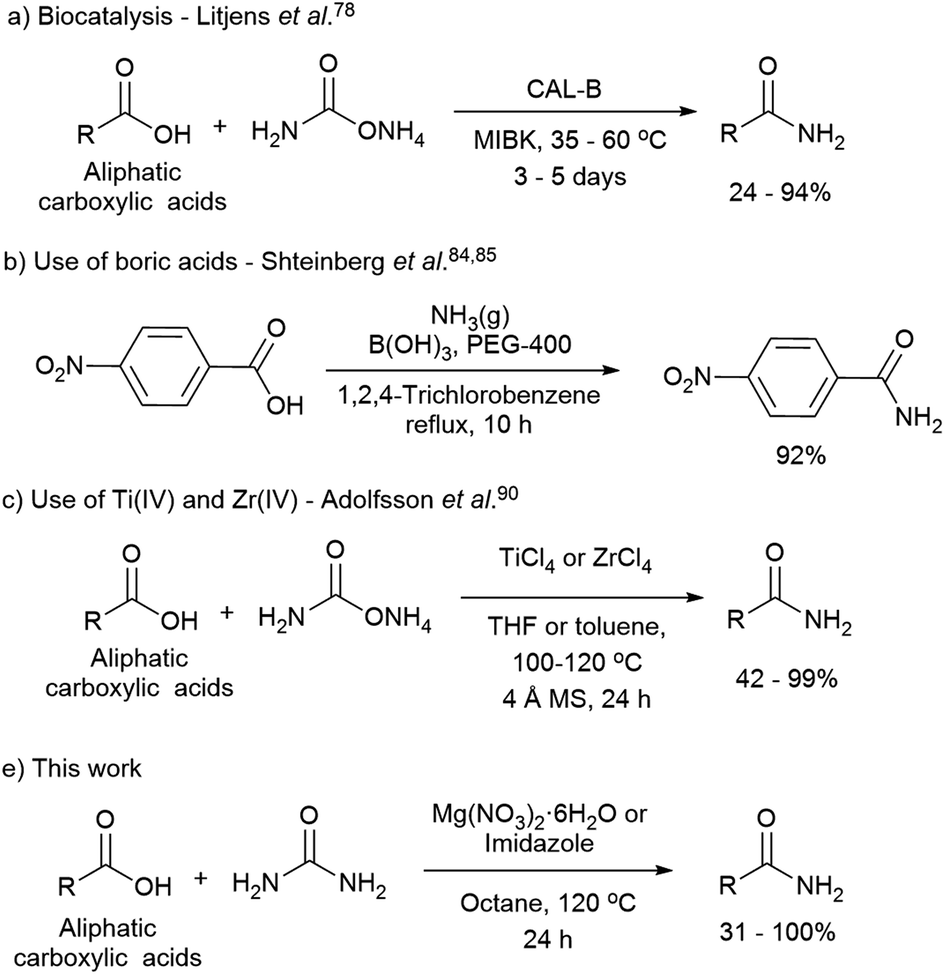
Direct Synthesis Of Amides From Nonactivated Carboxylic Acids Using Urea As Nitrogen Source And Mg No 3 2 Or Imidazole As Catalysts Chemical Science Rsc Publishing Doi 10 1039 D0sc01317j
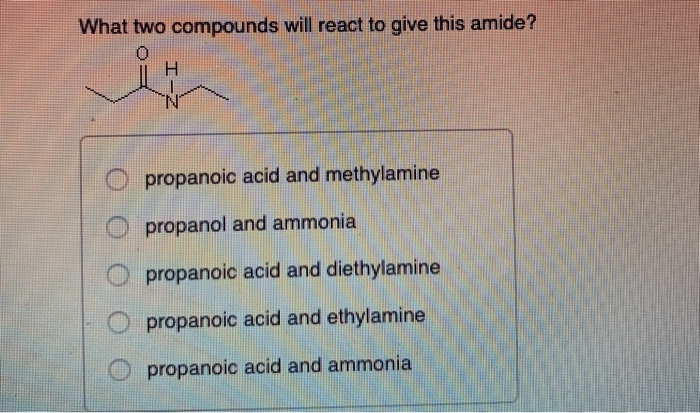
Solved What Two Compounds Will React To Give This Amide Chegg Com
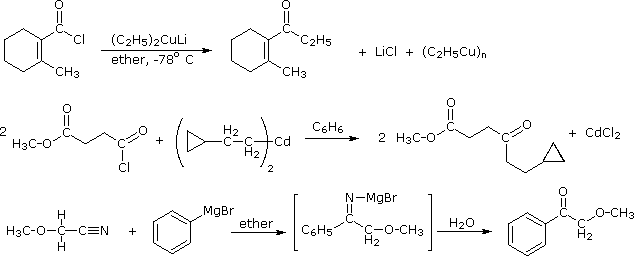
Carboxyl Derivative Reactivity
What Are The Reactions For Amines And Amides Example
Carboxyl Derivative Reactivity
Carboxyl Derivative Reactivity

Chemistry Chapters 13 14 Flashcards Quizlet
Solved Part A What Two Compounds Will React To Give This Chegg Com
Solved What Two Compounds Will React To Give This Amide On Chegg Com

Solved Question 13 What Two Compounds Will React To Give Chegg Com
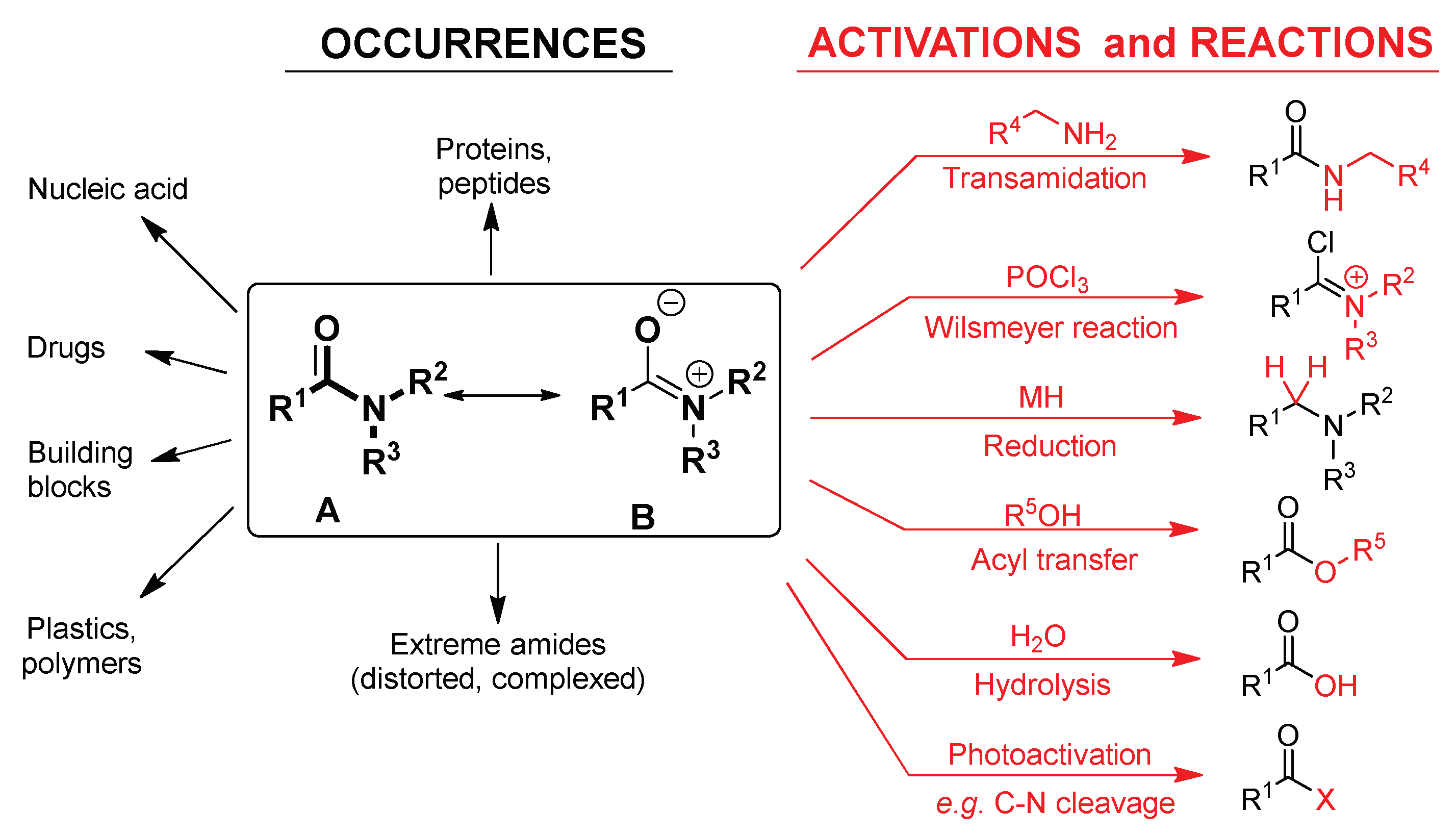
Molecules Free Full Text Amide Activation In Ground And Excited States Html
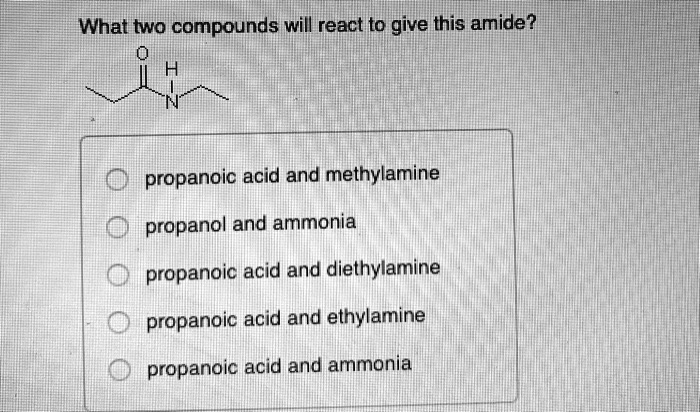
Solved What Two Compounds Will React To Give This Amide Mlpropanoic Acid And Methylamine Propanol And Ammonia Propanoic Acid And Diethylamine Propanoic Acid And Ethylamine Propanoic Acid And Ammonia

Solved Question 51 1 What Two Compounds Will React To Give Chegg Com
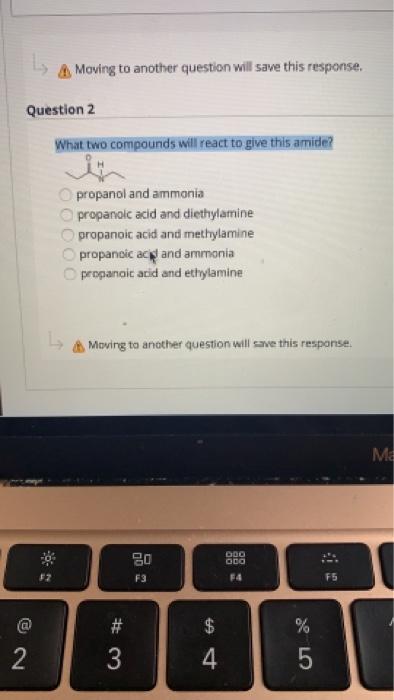
Solved A Moving To Another Question Will Save This Response Chegg Com
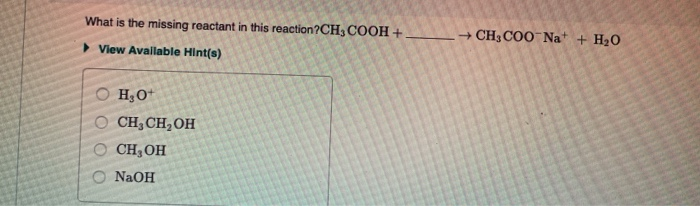
Solved What Two Compounds Will React To Give This Amide Chegg Com
Solved Item 13 Which Compound Below Contains An Ester Chegg Com

Comments
Post a Comment